A STUDENT'S GUIDE TO COLD FUSION,
by Edmund Storms, February 2003
FOREWORD
My interest in
cold fusion began shortly after Profs.
Pons and
Fleischmann announced their claims in 1989, while I was but an ordinary conventional research scientist working at LANL (Los Alamos National Laboratory). Of the numerous attempts to duplicate the claims, I was fortunate in producing
tritium as well as anomalous energy. There is nothing like seeing a phenomenon for yourself to make a person believe that it is real, regardless of what less observant people might claim. Also, seeing many fellow scientists acting foolish and self-serving provided an additional but disappointing education. Since retiring from LANL twelve years ago, I have continued to investigate the subject, to write papers, including several scientific reviews, and to lobby for acceptance of the phenomenon. The large collection of references, being nearly 3000, acquired in this effort was made the
LIBRARY on www.LENR-CANR.org. With essential help provided by
Dieter Britz and
Jed Rothwell, this collection of literature will be kept up to date as the field grows.
Literature on the subject of cold fusion has grown beyond a point where casual reading can lead to useful understanding. Although several good books are available, they do not address the scientific issues and the various scientific reviews are too narrowly focused.
This paper is designed to give a technically trained person an overall understanding of the claims and evidence for the effect in as condensed a manner as possible. Someone wishing a nontechnical understanding should read “Excess Heat: Why Cold Fusion Research Prevailed” by
Charles Beaudette [1] or the nontechnical section of www.LENR-CANR.org.
This paper is neither a complete review nor a critique of known information, but rather a guide. I have chosen only a sample of useful papers, with frequent reference to reviews where a more complete list can be found. Links to the complete LIBRARY on LENR-CANR.org allow full text papers to be immediately consulted. A complete list of references is attached so that this paper can be printed and viewed as an independent document. The cited references must be consulted for a complete understanding once the map provided here has been studied.
If the reader finds an important reference missing from either the LIBRARY or from this paper, please contact me at
Storms2@ix.netcom.com so that the oversight can be corrected. In addition, a section will be made available on the website to which critiques of this paper or additions can be submitted. It is my intention to encourage debate and to advance the field at all levels of belief and understanding.
When making suggestions, please bear in mind that many papers do not provide sufficient information to allow an evaluation of accuracy or a conclusion about what the observation means. This is especially true of many studies that failed to produce anomalous effects. Although skeptics often point to failures as a way to reject the process, actually a failure in one laboratory seldom casts doubt on work in another, unless the two use exactly the same instruments and techniques. Failure has many fathers besides the claim being false. Hopefully, this discussion will help future authors address these important issues, whether their work fails or succeeds. Without such information, I am frequently forced to state what was observed without the satisfaction of providing the reader with further insight.
New work has revealed several incorrect assumptions that have led research into unproductive directions. I suggest theories and future studies now take into account the following:
1. The effect occurs in the surface of an electrolyzing cathode, not in bulk material.
2. Active material causing the Pons-Fleischmann effect is not β-PdD of any composition, but is a complex compound of unknown but high composition and of unknown structure.
3. Nuclear reactions are found to occur in many materials treated in a variety of ways, and not just when palladium and deuterium are present.
4. An environment consisting of nanosized particles is very frequently observed when nuclear effects occur.
5. All isotopes of hydrogen can be involved in the cold fusion process.
In addition, I suggest that all past explanations based on the ideal properties of β-PdD must be abandoned as being hopelessly inadequate. Until the nature of the real world, in contrast to the ideal imagined world, is addressed by theory, the field will continue to stagnate. These conclusions, some my own and some shared by others, provide the basis for this paper.
GENERAL INTRODUCTION
The controversial phenomenon called "Cold Fusion" (CF), "Low Energy Nuclear Reactions"(LENR) or Chemically Assisted Nuclear Reactions" (CANR) involves the proposed ability to initiate a wide variety of nuclear reactions in solid materials using much lower energies than thought possible. Rather than using brute force to move nuclei to within reaction distance, apparently a mechanism exists in a lattice structure that is capable of circumventing any Coulomb barrier, allowing certain nuclei to interact. This paper will address the major observations that are used to support the claimed anomalous behavior. To help the reader obtain a quick overview of the claims, minimal detail is provided in the text. All of the many omitted papers are available in the website LIBRARY where dedicated readers can browse to their heart's content.
Chapter 1 gives an overview of the methods used to initiate the effect. Evidence for anomalous heat is summarized in Chapter 2 and nuclear products are discussed in Chapter 3. A few explanations for the nuclear mechanism are provided in Chapter 7. These anomalous nuclear reactions require a special environment in which to operate, the so-called Nuclear Active Environment (NAE). This environment is described in Chapter 4. Because duplication of the claims has been difficult for many people, some insights are provided in Chapter 6 to help in this effort. For a study to be useful, a student needs to understand the chemical properties of materials used in the attempt. These are described in Chapter 5 for only the Pd-D system where a few misconceptions are discussed. Finally, some plausible prosaic explanations and possible errors are offered in Chapter 8.
This discussion is designed to be a guide for amateurs and professionals alike. The claimed effects are accepted as being real, although not well understood or necessarily accurate in their reported magnitude. This paper intends to show important patterns of behavior, to suggest ideas that might have been overlooked, and to give a student some understanding of how to duplicate the claims. The reader can make the final judgement as to whether such a large and consistent collection of observations can be produced by error, chance, or prosaic processes.
CHAPTER 1: Overview
Palladium deuteride was once thought to be unique in its ability to host such reactions. Many other metals and metal alloys now have been found to produce the same novel effects. However, all of these anomalous effects are sensitive to the chemical environment in which they occur. Apparently, chemistry is as important as physics to this phenomenon, a fact that is frequently ignored.
The NAE has been generated many different ways and exposed to a range of applied energy from several sources. The first reported method
[2] used electrolysis to load palladium with deuterium. Electrolysis has also produced success using nickel cathodes with a H
2O containing electrolyte
[3, 4], platinum with D
2O
[5], and titanium with D
2O
[6]. Increased temperature
[7-9], applied RF energy
[10], and laser light
[11-13] appear to enhance the effects. Use of voltage sufficient to create plasma
[14, 15] in the electrolyte has been found to generate a variety of anomalous nuclear reactions when palladium, tungsten or carbon
[16] is used as the cathode. The kind of atom dissolved in the electrolyte and subsequently plated on the cathode plays a dominant role in determining which nuclear reaction occurs on the cathode. Thin layers of material plated on glass
[17], platinum
[18], and copper
[19, 20] have also become nuclear active when electrolyzed. Simply exposing finely divided metal of various kinds to hydrogen isotopes can generate anomalous effects. When exposed to deuterium gas, nanometer-size palladium particles become nuclear active. This palladium powder can be freestanding as “palladium-black”
[21] or attached to a carbon surface
[22], as in a conventional hydrogen catalyst. A flux of deuterium caused to pass through a 40 nm layer of palladium can also generate a variety of nuclear reactions
[23], depending on the kind of atoms dissolved in the palladium.
Energetic ions, obtained by discharge in gas containing hydrogen isotopes
[24, 25] or by ion bombardment
[26-35], have been used to initiate nuclear reactions. In all cases, ion energy is far below that thought necessary to cause a significant nuclear effect.
Certain complex metal oxides
[36, 37] are capable of dissolving some deuterium, which can be electrodiffused within the structure by applying a voltage. Anomalous energy has been generated using this method. Electrodiffusion of D
+ in β-PdD may also produce anomalous heat
[38-42].
Bubbles generated by sonic energy passing through a liquid can collapse on a metal surface. When this happens, the bubble content is injected into the metal as plasma. Use of heavy water injects a mixture of D
+ and O
--, which produces anomalous nuclear products and heat in a variety of metals used as the target
[43-45]. Normal water may produce similar novel effects, although duplication has yet to be successful
[46].
Anomalous effects have been seen during a variety of chemical reactions when deuterium is present
[47, 48]. Sudden heating of titanium charged with D
2 [49] or cooling of titanium in D
2 gas
[50, 51] results in neutron emissions. Many chemical reactions involving deuterium have been reported to generate neutrons, including the setting of Portland cement. Nuclear effects have also been reported to involve biological systems in the presence of both D
2O
[52] and H
2O
[53, 54]. Although the number of nuclear events is small in these environments, conventional theory would have none produced.
Only a few studies have measured nuclear products at the same time as anomalous energy. These measurements show a direct relationship between energy and 4He production when deuterium is present, as described in Chapter 3 and a relationship between transmutation products and heat. On the other hand, tritium or neutron emissions are seldom associated with detected heat, although occasionally X-ray emissions are observed. Apparently, the path taken by the fusion reaction is much different in a lattice compared to when energetic plasma is used.
Hydrogen is also found to be nuclear active in some environments. Anomalous effects are produced by specially treated nickel surface when exposed to hydrogen gas
[55], Nickel, when it is repeatedly loaded and deloaded using hydrogen gas, appears to produce tritium
[56]. Hydrogen can also produce transmutation products and detectable energy
[57-65]. Even tritium, when reacted with finely divided titanium
[66], experiences a change in its decay rate.
This is only a brief sample of conditions reported to produce strange effects, many of which have been done with enough care and duplication to support the claims. Only a few of the many duplications are noted here.
Unfortunately, the nature of the NAE has been difficult to discover because the reactions only occur in very small regions that have properties much different from the surrounding bulk material. More detail will be provided in later chapters.
New methods are being explored and old methods are being replicated. Skeptics predicted that cold fusion was an artifact that would disappear when better instruments and techniques were used, but this has not happened. On the contrary the effects have been more widely reported at higher signal-to-noise ratios. Clearly, the unique mechanism can be initiated many different ways, in many chemical structures, and involve all isotopes of hydrogen. The challenge is to determine what these structures and mechanisms have in common, not to reject them because they are novel.
CHAPTER 2: Energy Production
I. Explanation of the Calorimetric Method
Demonstration of energy production requires use of a calorimeter. Several kinds have been used, which include isoperibolic, flow-type, and Seebeck. Because calorimetry is a mature science, its errors and limitations are well known. However, not all measurements of the LENR effect have taken advantage of this knowledge. Anyone attempting such measurements or evaluating the claims must first learn what is known about the method being used, as described below.
Isoperibolic calorimetry uses the temperature difference across a thermal barrier to determine the amount of thermal power being generated within the barrier. Accuracy depends on ΔT being known over the whole barrier area and remaining stable. Errors can be introduced when the wall of an electrolytic cell is used as the thermal barrier and temperature is measured within the electrolyte. Unexpected temperature gradients are usually present, which compromise the measurement. Under these conditions, accuracy depends on design of the cell, location of the temperature sensors, and stirring rate.
[67] This method requires suitable calibration, usually by electrolyzing an inert electrode. Use of an internal heating element for calibration is not recommended, especially in the absence of stirring or simultaneous application of electrolytic current. A refinement of this method uses a thermal barrier external to the cell
[68, 69]. Such a design is much less affected by gradients within the cell and can be made very sensitive to generated thermal power.
Flow-type calorimeter captures released thermal power in a flowing fluid and measure the resulting temperature change of this fluid. If no energy is lost from the calorimeter, the amount of thermal power can be obtained using flow rate, temperature change, and heat capacity of the fluid, the so-called absolute method. However, complete capture of all energy is very difficult. Consequently, the calorimeter must be calibrated by using an internal heating element or by electrolyzing an inert electrode. The advantage of this method rests on it being relatively insensitive to where energy is generated within the cell. However, isolating the calorimeter from the environment and achieving a constant, known flow rate can be a challenge.
A Seebeck calorimeter generates a thermoelectric voltage produced by the temperature difference across a thermal barrier containing thermocouples. This barrier completely surrounds the heat source, with the outside kept at constant temperature. Because all parts of the surrounding wall contain the same density of thermocouples connected in series, loss of energy through each part of the thermal barrier is summed, regardless of where heat energy leaves. Unfortunately, not all locations are completely equivalent. As a result, the calibration constant is sensitive to where the heat source is located within the thermal envelope. This problem can be reduced by instillation of a fan. On the other hand, this method is insensitive to where heat is generated within a cell containing the heat source. Only the position of the cell must be kept constant within the thermal envelope. This method also must be calibrated.
Many variations on these methods have been used, some with good success. Reliable measurement of anomalous power of ±50 mW, superimposed on 15 watts of electrolytic power, can be routinely achieved. Some special designs are reliable below 1 mW when less electrolytic power is applied.
II. Anomalous Energy
II.1. Electrolytic Method
The first claim for anomalous heat generation was provided by Pons and Fleischmann
[2], using electrolysis and an isoperibolic calorimeter. This work was subjected to considerable analysis and debate, but was eventually found to be sufficiently accurate to support their claim
[70]. Since this work was published, well over100 claims for anomalous energy using electrolysis have been published, many finding more than one sample to be active. Unfortunately, only about 37 of these publications provide enough information to allow an analysis of possible errors. Most of these studies measured several samples of palladium, with some being active and some inactive. These reports are tabulated by Storms
[71], who also evaluated the suggested prosaic explanations.
Until recently, anomalous energy was assumed generated within the β-PdD structure. Many recent observations indicate that only small regions in the surface are active and these turn rapidly off and on
[72]. Presumably, a region starts to generate energy, heats up, expels deuterium, and turns off. Rapid repletion of the process produces apparent steady energy. Occasionally energy density is sufficient to cause local melting. This region consists of a complex alloy containing many elements, but little palladium. More will be said about this situation below.
When the electrolytic method is used with a palladium cathode, everyone who makes suitable measurements always sees six characteristic behaviors. These are:
1. The average D/Pd ratio of the entire cathode must exceed a critical value. This value differs somewhat between studies because only the average composition can be determined, which depends on the method used and the shape of the cathode. Typically, the average critical value lies between D/Pd=0.85 and 0.90. Infrequently, compositions above this range are found to be inactive for unknown reasons. The actual composition of the active surface appears to be above D/Pd=1.5 and perhaps as high as D/Pd=2.0
[73, 74], as shown in Fig 1. Failure to reach a sufficiently high composition on the surface, regardless of the average composition, can explain occasional failure of highly loaded samples.
FIGURE 1. Measurements of thin film composition as being representive of the true surface composition.
2. Current must be maintained for a critical time. This time is variable and presumably depends on how rapidly the surface can acquire the active structure and/or composition. This time is short for very thin layers of Pd, while it can be as long as months for bulk palladium. Failure to wait the necessary time is one reason some people have not seen the effect.
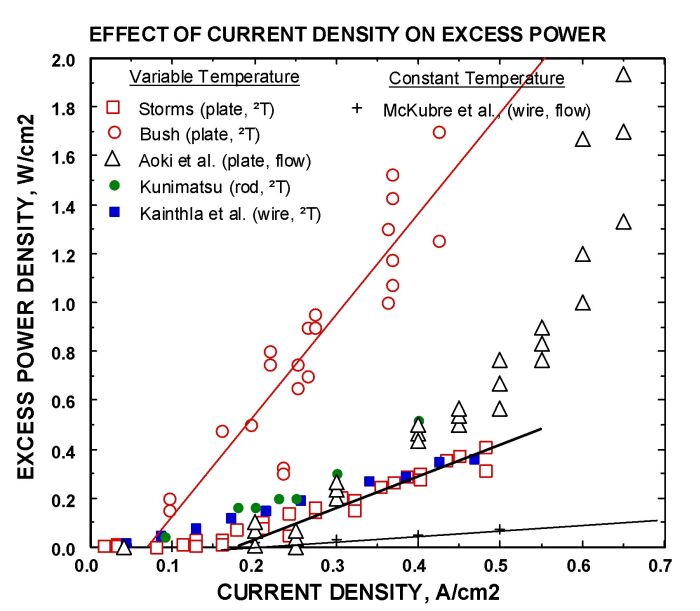
3. Current density must be above a critical value, as shown by a few examples in Figure 2. Applied current determines the surface composition, hence the nature of the active structure. A value above 150 mA/cm2 is usually found for bulk palladium. Presumably, currents above this value are required to compensate for the loss of deuterium from the backside of the active surface. Thin layers of palladium deposited on platinum do not require such a high critical current because backside loss is trivial, provided the layer is well bonded. These samples show anomalous energy at currents near zero.
FIGURE 2. Examples of the effect of current density.
4. Inert palladium can sometimes be activated by addition of certain impurities to the electrolyte. These impurities are proposed to help the surface achieve a higher deuterium content and/or suitable structure.
5. The effect occurs in only a small fraction of samples, but more often in certain batches than in others
[75]. This is consistent with the fact that all physical properties of palladium are found to be batch specific, making this metal highly variable in its general behavior, even in conventional applications. Electroplated palladium has a higher success rate, although it also can be highly variable depending on conditions used during plating.
6. Presence of too much light water in the D
2O electrolyte will stop the reaction
[76]. Heavy water is highly hygroscopic so that exposure to laboratory air will quickly render the material useless. This oversight explains many early failures.
In a few cases, the same batch of active palladium was studied in different laboratories
[76]. On one occasion, the same active sample was studied in different laboratories
[77]. Anomalous heat was obtained in each case. In fact, the author has found that once an active cathode is produced, it can be used to produce anomalous results at will, with total reproducibility.
The electrolytic method has met all of the criteria science requires to accept anomalous claims. Anomalous heat production has been independently duplicated many times with values frequently far in excess of expected error, the results show the same patterns of behavior regardless of the apparatus used and reasons why duplication is difficult have been identified. However, the source of anomalous energy is not revealed by such studies nor is knowing its source required to accept the observations. Later chapters will explore evidence supporting a nuclear source.
II.2. Gas Loading Method
Arata and Zhang
[78] at Osaka University in Japan were the first to generate anomalous energy using finely divided palladium. This powder is contained in a palladium capsule, which is pressurized with very pure deuterium, generated by electrolysis. The claim was duplicated at SRI
[79, 80] with Prof. Arata's help.
After this work was published, Case heated a commercial palladium catalyst in deuterium gas and reported anomalous energy and helium. This claim was also duplicated at SRI
[79] with Case's help.
Both results are difficult to replicate because characteristics of the active material are critical, especially particle size and purity.
Recently, Iwamura
et al. [23] at Mitsubishi Heavy Industry in Japan deposited a thin layer of palladium (40 nm) on a layer of CaO, which had been deposited on palladium. When deuterium was caused to diffuse through this sandwich, several nuclear reactions were detected, including excess energy
[81].
II.3. Electrodiffusion Method
Electrodiffusion is a process whereby ions dissolved in a material are caused to move under the influence of applied voltage. The enhanced diffusion rate is proportional to applied voltage and to the amount of charge on the ion, thereby allowing the effective charge of a dissolved ion to be determined
[82]. The effective charge on hydrogen in PdH
0.67 is +0.30±0.05
[83], with an apparent increase in positive charge at higher H/Pd ratios
[84, 85].
This method was first applied to an oxide environment by Mizuno
[36] and later duplicated by Prof. Oriani
[37] with Prof. Mizuno's help. Preparata
et al. [86] first applied the method to palladium deuteride, at which time it was called the Coehn-Aharonov Effect. Workers in Italy have further refined the method
[87, 88]. In each case, anomalous energy is produced.
The net effect of ion diffusion driven by applied voltage and normal diffusion driven by a concentration gradient may be identical as far as the nuclear mechanism is concerned. Consequently, a flux of deuterium ions that is almost always present may aid in energy production, as suggested by McKubre
[89], just as does an applied voltage.
II.4. Sonic Method
Stringham
[90], with the assistance of George, pioneered use of the sonic method to load solid metals with deuterium. Other workers have tried loading materials suspended within D
2O
[91]. Evidence for nuclear products was reported in each case and anomalous energy in the former study. Recently, Prof. Arata has replicated the results in Japan using a similar method. However, the method has proven difficult to replicate by other people. This method is not the same as used by Taleyarkhan
et al.
[92] to generate neutrons
within the collapsing bubbles. In their case, temperatures of thousands of degrees, reached just before the bubble vanishes, may produce a brief “hot” fusion process, but not “cold” fusion.
A number of reports of anomalous heat using mechanically generated cavitation have been reported using light water
[46, 93]. These claims have not been replicated, although attempts have been made.
CHAPTER 3
Nuclear Products
I. Introduction
Once claims for anomalous energy are accepted, identification of its source is the next problem. Because of its large magnitude and the absence of an obvious chemical source, Pons and Fleischmann suggested the energy came from fusion of two deuterons. This suggestion immediately got them into trouble with the physics community.
The fusion reaction has three potential paths shown below. Each path contributes the indicated fraction when fusion is caused to occur at high energy, as in plasma — in other words, as “hot” fusion. The branching ratio between the neutron and tritium paths is energy independent above 20 keV, but may be sensitive to applied energy at lower energies
[94] and to the chemical environment as well
[95].
Nuclear reactions resulting from fusion of deuterium
| Reaction Energy | MeV | Fraction |
| d + d = Helium-4 + Gamma | 23.9 | <0.01 |
| d + d = Tritium + Proton | 4.03 | 0.5 |
| d + d = Helium-3 + Neutron | 3.27 | 0.5 |
These branches were initially thought to occur with the same fraction when anomalous energy was made in a P-F cell. Consequently, early rejection of the claims was based on the many studies that failed to detect significant neutron emission or tritium production.
Search for a nuclear product then focused on 4He and was rewarded by early success. This work also is rejected because the required gamma emission is absent. Helium-4 can not be produced from simple fusion unless the momentum of the two reacting nuclei can be shared between two emitted particles, in this case a photon and a helium nucleus. Suggestions that this energy might be shared with atoms or electrons in the environment are also rejected because the time needed for this transfer is considered too great compared to the extremely fast energy release from a fusion reaction. Nevertheless, evidence for helium production continues to accumulate.
The amount of detected tritium is never enough to account for observed energy and it is seldom detected at all even when anomalous heat is being made. Nevertheless, it has been observed many times, and it is clearly anomalous even when the amount is small.
Tritium detection is a mature science that is able to measure concentrations well below those found in cold fusion cells, a fact that eliminates measurement error as an explanation. Initially, tritium was dismissed as being caused by contamination from the environment. Use of sealed cells eliminates this possibility. Palladium was proposed to contain tritium as a leftover from its assumed use in weapons production. Careful analysis of commercial palladium and use of virgin material eliminates this possibility
[96]. The normal tritium content of D
2O can be concentrated by electrolysis when a recombiner is not used. While this effect might explain a few observations, it can not explain them all, because most successful studies now use a recombiner. Fraud was even suggested
[97] in a futile attempt to discredit work at Texas A & M University
[98]. At the present time, no plausible prosaic process explains the occasional increase of tritium in sealed cells containing a recombiner. Although many chemical environments have been explored, no NAE has been identified to which tritium production can be related.
Neutrons are detected, usually as bursts, but the rate suggests this fusion path is the least used by the CF process. Indeed, neutrons might not even result from cold fusion at all. A process called fractofusion has been suggested whereby cracks produced within a material can generate sufficient voltage gradient and/or temperature within the crack to initiate a local “hot” fusion reaction
[99-101].
Even though most of the nuclear energy disappears as heat, some is retained as emitted energetic particles and electromagnetic radiation. However, the amount of radiation and its energy are much less than expected based on the behavior of “normal” nuclear reactions.
Recently, and with great difficulty, evidence for nuclear reactions other than fusion is accumulating. These are called transmutation reactions and involve elements much heavier than hydrogen. They are found to occur in many environments, including living cells, when a variety of methods are used. Indeed, the more often these reactions are sought, the more often elements are found in unexpected amounts and/or with abnormal isotopic ratios.
II.1. Helium Production
Helium is measured using a high-resolution mass spectrometer. A major error involves the possibility of air, which contains 5.6 ppm 4He, being mixed with the analyzed gas. Such contamination is revealed by the presence of argon in the gas, which is present in air at 0.94%. A memory effect in the mass spectrometer can distort measurement if care is not taken to flush out previously admitted helium. Deuterium gas (D2), which has a mass very close to helium, is removed chemically before the remaining gas is submitted to the mass spectrometer.
Helium is expected to reside in either the surrounding gas or in the metal structure. When helium is generated within a metal structure, it can only be removed by heating the metal near its melting point
[102]. Because at one time bulk palladium was thought to be active within its entire volume, helium was extracted from the entire palladium cathode and analyzed by mass spectrometry. This work is summarized in a review
[103]. The observed helium, although anomalous, has been attributed to either air contamination during analysis or ubiquitous dissolved helium. Later, measurements were made of helium present in gas evolving from electrolytic cells. At least five independent measurements
[104-108] show a relationship between amount of energy and helium produced. Recently, helium also has been detected after energy production in gas loaded cells containing finely divided palladium
[79, 109]. Because most helium appears in the gas rather than in the metal, it is safe to assume that helium is produced very near the surface rather than in the bulk.
II.2. Tritium Production
Tritium is radioactive, decaying by beta emission to
3He with a half-life of 12.3 years
[110]. Tritium is normally detected by placing it in an organic fluid that gives off light upon passage of the beta particle. This light is detected by a photomultiplier tube and presented as an energy spectrum and total number of events. Chemiluminescence, i.e. light produced by a chemical reaction, is a potential source of error that can be eliminated by waiting for a suitable time or vacuum distilling the sample. The accumulated
3He can also be detected using a mass spectrometer or the beta current can be measured using an ionization cell and a sensitive electrometer. Because the emitted beta particle is barely able to pass through a piece of paper, direct detection can be difficult. Although detectable tritium is present in the normal environment, a residue from atom bomb tests, the amount is much less than that found in CF cells.
Tritium has been produced using several different techniques, including electrolysis, gas loading, and ion bombardment. In each case, success is very dependent on the material used. Of these, the electrolytic method has been given the greatest attention. Electrolysis concentrates tritium that is always present in commercial heavy water. Therefore, either a sealed cell, containing a recombining catalyst, must be used or the evolving gas must be collected and analyzed separately for tritium. Many studies have calculated the increased amount of tritium expected from the known separation factor
[111, 112] and subtracted this amount from the measurement. This method is the least accurate of the three, but satisfactory when large amounts of tritium are found. Three studies deserve special attention because of the unique understanding they provide. A summary of other measurements is given in a review by Storms
[103].
II.2.1 Electrolytic method
Will
et al. [113] used sealed glass cells containing a recombiner, so that environmental contamination was not possible. To evaluate this possibility, an identical cell containing H
2O was run at the same time using material from the same batch of palladium. These control cells never showed any increase in tritium content. Tritium analysis showed more tritium in the electrode than in the electrolyte. This can only happen when tritium is generated within the electrode because deuterium quickly displaces tritium from palladium during electrolysis
[114]. Similar pieces of palladium from the same batch were analyzed and shown not to contain tritium
[96]. No plausible source of tritium has been suggested to explain these observations. The amount of anomalous tritium was far in excess of the sensitivity and error of the detector. Matsumoto
[115] also found tritium when a D
2SO
4electrolyte was used with a palladium cathode.
Storms
[114, 116] showed how tritium behaves in an electrolytic cell. Tritium contained initially in the palladium cathode as contamination is quickly released by electrolysis and appears in the evolving D
2 gas. On the other hand, tritium that results from the CF process appears in the electrolyte, with much less in the gas. This behavior eliminates tritium dissolved in the electrode as being the source of anomalous tritium in the electrolyte when cells are used without a recombiner and is consistent with tritium being produced on the surface of the cathode during the CF process. Of course, when a recombiner is used, the evolving gas is converted to D(T)
2O and mixed with the electrolyte, thereby making a distinction between the two sources impossible.
Bockris and his students
[117] found tritium in a cell lacking a recombiner and using a palladium cathode and D
2O. Shaking the cell could stop tritium production and increasing cell current (voltage) could increase the production rate. Copper, from an exposed wire, was found on the cathode after the study. This was thought to occur as dendrites, removal of which upon shaking was thought to interrupt production.
Anomalous tritium has also been reported in light-water cells containing a nickel cathode
[118].
II.2.2 Ion bombardment method
Claytor
et al. [24], in a well documented and through study done at LANL over many years, generated tritium by subjecting certain alloys to pulsed discharge in D
2 gas at modest voltages (<7000 V). The amount of tritium generated depends on the material used as the cathode, with complex alloys being more productive than pure palladium.
II.2.3 Gas loading method
Clarke
[80] detected
3He in an Arata-type cell provided by McKubre
[79], which could be explained by decay of tritium produced during the initial study. During this study, the palladium cell containing palladium-black was loaded with very pure D2 gas generated by electrolysis. Nothing else was done to the cell and the quantity of
3He was not consistent with tritium being present before the study. Itoh
et al.
[119] loaded palladium with deuterium, coated the material with copper, then deloaded by heating in vacuum. Tritium emission increased substantially when the initial average composition was over D/Pd=0.85.
Tritium was found in nickel wires after being electrically heated and cooled many times in hydrogen
[118]. The resulting hydride layer, in which tritium was found, was 20-30 nm thick
II.3. Neutron Production
Neutrons are detected using several types of counters, including those containing
3He or BF
3 gas. Neutrons reacting with these gases produce bursts of energy that are detected as voltage pulses. An energy spectrum can be obtained using NE213
[120] or Li-glass scintillation
[121] detectors, which detect the gamma ray emitted when a neutron reacts with lithium within the detector. Because the number of neutrons emitted from a CF cell is so small, great care must be taken to eliminate false counts produced by electrical discharge, Cosmic rays, or normal sources in the environment
[122-124]. Occasionally, very large bursts are seen for a brief time. These bursts are seldom associated with measurable heat or tritium production. When they are, the n/t ratio is as small as 10
-9.
Most attempts to detect neutrons fail, thereby adding to the skepticism. However, a few studies noted here give interesting insight about the mechanism for their generation. A more complete summary can be found in the review by Storms
[125].
Takahashi
et al. [32, 126, 127] measured the energy of neutrons emitted from electrolytic cells containing a palladium cathode subjected to alternate high and low applied current. Neutrons were seen at 2.54 MeV and between 3-7 MeV, suggesting a multi-body process for their production. This idea was further explored using ion bombardment.
Scaramuzzi and co-workers
[128] reported neutron emission when titanium was temperature cycled in D
2 gas. This observation prompted many attempts
[129] to replicate the claim, with many being successful, but with many failures. Analysis after a similar study showed the presence of anomalous tritium
[130]. Emission appeared to occur most often when titanium passed through a temperature associated with a phase change. Considerable cracking of the hydride occurred, but simple crack formation did not seem to relate to emission. Jones
et al. have recently detected neutron emission from titanium electrodes during electrolysis, similar to the claims made by these workers in the past
[131].
II.4. Energetic Radiation
Occasionally, low-energy radiation detectors are placed on or near an active surface either during or after the study. Evidence for low-energy X-rays of various frequencies is sometimes obtained
[132-144]. When energy is measured, it can be sometimes attributed to characteristic K-alpha emission from atoms known to be present. Occasionally, the radiation appears to result from radioactive decay. Evidence for tightly focused beams of radiation has been reported
[106] from electrolytic cells as well as during ion bombardment
[28]. This behavior is important because it indicates that emitted radiation can be sensitive to the physical orientation of the source, much like a solid-state laser. Particle detectors, such as CR-39 plastic
[28, 145-155], placed near an active surface show evidence for energetic alpha and proton particle emission as well as energetic electrons, although not all from the same sample.
Energy is being generated and released as electromagnetic radiation and particles, as expected, but this energy is too low to allow escape from the apparatus. This is a mixed blessing because it allows studies to be made without danger of radiation exposure, but it makes this diagnostic tool more difficult to use. It also shows that all of the nuclear energy is not immediately communicated to the lattice, but is retained by some nuclear products.
II.5. Transmutation Products
Transmutation products consist of elements much heavier than hydrogen. These are detected using various methods including neutron activation, XPS, EDX, and SIMS. Occasionally sufficient material is produced to allow normal chemical analysis.
Most evidence is based on using the electrolytic or gas discharge methods, or a combination thereof. Unexpected elements seem to result from many kinds of reactions, including fusion between any hydrogen isotope and a heavy element, fusion between two different heavy elements, and fission of a heavy element. Abnormal isotopic ratios are frequently found. Only a few of the many reports are described here.
Miley
et al. [59, 156] have studied this process in some detail using electrolysis of mainly electrolytes based on H
2O. A spectrum of nuclear products is found, with high concentrations falling into four mass ranges of 20-30, 50-80, 110-130, and 190-210
[157]. Mizuno
et al. [158, 159] have also explored the subject in detail using mainly electrolytes based on D
2O. Abnormal isotopic ratios of Hg, Fe and Si were found. Although some minor elements might have resulted from contamination, it is very difficult to understand how major concentrations could come from this source, especially those having abnormal isotopic ratios.
Compounds dissolved in an electrolyte can deposit their positive constituent on a nickel cathode where it has been found to be converted to another element. For example, when potassium compounds are used, calcium is formed when H
2O is present
[63, 160, 161]. Other similar elements suffer the same fate in H
2O
[162, 163]. Cathodes made from other metals produce a more complex result
[164].
A particularly compelling study was reported by Iwamura
et al. [23]. They deposited 40 nm of palladium on a layer of CaO, which had been deposited on bulk palladium. A small amount of Cs or Sr was applied to the surface by electrolysis. When deuterium was caused to diffuse through this sandwich, a reduction in the amount of initial element and a growth of Pr or Mo was observed by XPS, respectively. The Mo had an isotopic concentration like that of Sr, not like normal Mo. This work shows that transmutation reactions can occur by addition of 4 deuterons to the target nucleus as a single event. Why Pd was not transmuted needs to be explained.
Evidence for iron production during arcing between carbon electrodes under H
2O has been reported
[16, 165-167]. This method seems to be easily reproduced. Palladium and gold cathodes also showed excessive iron after electrolysis in light water
[168, 169].
Radioactive isotopes, other than tritium, are seldom reported. When they are, their presence is difficult to reject especially when their half-life is short. Bush and Eagleton
[170] produced a mixture of radioactive isotopes with an average half-life of 3.8 days in an electrolytic cell. Mizuno
et al. [159] found what appeared to be
197Pt after electromigration of D
2 in a solid oxide. Notoya
[62] found evidence for
24Na in an electrolytic cell containing Na
2CO
3 and H
2O using a Ni cathode. Gamma emitters were also found after ion bombardment
[171]. Wolf
[172] obtained a complex spectrum of gamma emissions after electrolyzing a cell containing D
2O with some Al, Ni, and B present. It is safe to conclude that radioactive elements may have been produced in other studies as well, but were not detected for lack of trying.
One of the most surprising and difficult to explain observations involves transmutation reactions within living cells. Such claims were made decades ago
[173], but only recently have the necessary careful measurements been done to give some credence. Vysotskii
et al. [174] showed that
55Mn is converted to
57Fe when a bacteria is grown in D
2O containing MnSO
4. Other anomalous nuclear reactions were discovered during later work
[175]. Komaki
[53] demonstrated that several types of yeast and bacteria grown in normal water convert elements in their environment to the ones they need when the required elements are absent. This provides one more unusual environment that must be addressed by theory, and one more strange observation that tests the ability of the reader to remain open-minded.
CHAPTER 4: Descriptions of the nuclear active environment
A number of observations place the NAE in the surface region of a Pons-Fleischmann cathode. These are:
1. Almost complete loss of helium to the gas.
2. Appearance of tritium in the electrolyte rather than in evolving gas.
3. Observed heat generated on the surface.
4. Transmutation products located only in the surface region.
5. Presence of melted regions on the surface.
6. Ability to generate a large effect using thin deposits on an inert substrate.
Many careful analysis of such surfaces reveal a complex alloy containing lithium, platinum, elements provided by the Pyrex container, and impurities in the electrolyte, with much less or no palladium. In addition, measurements of the surface composition place it above D/Pd=1.5, as can be seen in Figure 1, and perhaps as high as D/Pd=2. As argued by Storms
[72], the NAE is not pure β-PdD in an electrolytic cell nor may this phase be involved at all. At the very least, the material contains a much higher deuterium content than previously thought, which allows for the presence of deuterium dimers.
Other materials, including titanium and aluminum can support a NAE. Loading of aluminum with deuterium followed by electron bombardment is found to produce anomalous nuclear emissions
[147]. Even production of aluminum by electrolysis from cryolite appears to create tritium
[176]. Titanium when electrolyzed
[6] or when gas loaded by D
2 produces evidence for nuclear events in the presence of nanocrystals
[177]. Storms
[18]incorporated fine powers of various materials, including TiO
2 and Cs
2O, in palladium films and observed anomalous heat. Apparently, a great many compounds and structures can produce the NAE, with little evidence for β-PdD being one of them.
On the other hand, palladium-black
[21] and catalytic palladium
[22] host the NAE when exposed to modest pressures of deuterium. However, the impurity content, D/Pd ratio, and structure of these nanoparticles are not known. Until this information is available, the role of beta-PdD is unknown even in this form.
The only feature common to most, if not all, studies is the presence of nanosized particles when anomalous effects are observed. These structures are either provided initially or they are generated
in situ on a cathode surface by electrolytic action. Sputtering during ion bombardment can also generate them. Repeated loading and deloading of palladium
[178] or nickel will generate such structures as the material cracks. Energetic emissions are generated by this process
[143]. Jiang
et al.
[179] suggest that the nuclear reactions occur at the tips of such structures. Until the exact nature of the NAE is determined, reproduction of the effects will be controlled largely by chance and explanations will have little relationship to reality.
CHAPTER 5: Understanding how Pd behaves, including relevant properties
I. Introduction
Most metals react with the isotopes of hydrogen forming compounds. These compounds are either ionic (like LiH), metallic (like PdH), or covalent (like CH4). Ionic hydrides release hydrogen when exposed to water, while metallic hydrides are inert. Ionic hydrides contain hydrogen as a negative ion, while hydrogen is partically ionized to a positive ion in the metallic hydrides. Some elements such as silver, gold, and platinum form hydride only at very high-applied pressures. Nickel is difficult to hydride because a diffusion barrier of the hydride is formed on the surface. However, repeated cycling will eventually break up the structure and allow conversion. Palladium is not unique in its ease of hydride formation nor in the amount of hydrogen it can contain compared to many known alloys.
When beta-PdD is formed, the structure is cracked by the resulting large expansion
[180], resulting in many dislocations
[181]. As a result, preannealing of palladium is expected to have little effect on the resulting PdD. These cracks are hard to notice, but critical in determining the loading limit
[182-184]. Most elements, like uranium and titanium, turn to powder during the process, which renders them difficult to study in this context.
The properties of palladium hydride can be modified by alloy formation. Addition of silver prevents cracking, but reduces hydride stability. As a result, the same applied D2 pressure produces a smaller D/(Pd+Ag) ratio. Alloying with lithium increases hydride stability. Both elements substitute for palladium and cause a reduction in lattice size. Addition of platinum has an effect similar to silver. Boron, which substitutes for deuterium, makes the hydride more stable, but can result in brittle material if the very stable boride is allowed to form in the grain boundaries.
II. Properties
II.1. Phase diagram of the Pd-D system
Like the palladium-hydrogen system
[185-188], the palladium-deuterium system is known to contain two stable phases, an alpha phase created by deuterium randomly located between the palladium atoms (a typical solution) and a beta phase created by deuterium randomly located within a face-centered-cubic structure (a typical defect compound)
[189]. The lower phase boundary of this phase depends on applied D
2 pressure and temperature, as shown by Fig. 3
[190], but is at PdD
0.7 under 1 atm D
2 and room temperature. Above about 275° C, the alpha and beta phases merge into a single-phase region when more than 35 atm of D
2 pressure is applied. Deuterium occupies equivalent random sublattice sites in β-PdD above about 50 K. Below 50 K, ordered occupation between the occupied and unoccupided sites occurs
[191, 192]. The upper boundary is difficult to reach because very high-applied pressure is required. Presumably the beta phase can not exist above PdD
1.0. However, electrolytic action is able to generate sufficient chemical activity to drive the surface composition well above 1.0. However, application of modest pressures to palladium having a particle size below 5 nm results in compositions near D/Pd = 2.0
[250, 251]. One can only speculate about the resulting phase. However, other metals that form hydrides more stable than β-PdH, hence stable at accessible pressures, form compounds having limiting compositions of MH
2 and MH
3, some of which contain hydrogen dimers. Calculations indicate that dimers do not form in the β-PdH structure
[193]. In other words, the tetrahedral sites are not occupied. No evidence exists for the proposed gamma phase
[194].

FIGURE 3. The relationship between pressure and composition within the Pd-D system at various temperatures. The alpha-phase is on the left and the beta-phase is on the right. They come together as a single phase above about 275ºC and 35 atm.
II.2. Structure and lattice dimensions
Both alpha-PdD and β-PdD are face-centered-cubic, with the latter having a NaCl-type structure. Position of D atoms in the alpha-phase is assumed to be the same as those in the beta-phase, but with many fewer D atoms in these positions. However, location of the D atoms at a concentration available at room temperature has not yet been possible. Consequently, measurements were made at higher temperatures where the solubility is greater. Deuterium is presumed to have the same position at lower temperatures.
Conversion of alpha-PdD to β-PdD results in a volume increase of about 10%, thereby accounting for the tendency to form cracks. the amount of crack formation can be determined by comparing the expected lattice volume, based on the composition, to the measured volume
[114]. In forming the β-phase, atoms of Pd shift to allow D atoms into equivalent positions, as shown in Fig. 4. Hydrogen lies in the plane, forcing Pd atoms further apart and occupying the so-called octahedral position. These positions can be filled or empty in a random manner up to a completely filled lattice at PdH
1.0.
Because X-ray diffraction is insensitive to the position of hydrogen, X-ray patterns of the two phases appear to be identical, with only a shift in line position caused by different Pd atom spacing. Only neutron diffraction of Pd-D is able to determine the hydrogen position. A tetrahedral position is available, but neutron diffraction studies show that it is not occupied at compositions available to the studies
[195-197]. The resistivity behavior leads to the same conclusion
[198]. A study using low incident angle X-ray diffraction from an electrolyzing surface shows no evidence for unusual close approach between deuterium atoms
[199] and no new phases have been detected up to PdD
0.9 [200]. No evidence exists for tetrahedral occupancy, which is often assumed to be available.
Metastable structures have been obtained by quenching from 600° C while under high-pressure
[201] and by bombarding thin films of Pd with protons at 600° C, followed by rapid cooling in H
2 [202, 203]. Because these phases are metastable at room temperature and form under conditions not present in CF cells, they are expected to play no role in the CF process.
FIGURE 4. Figures showing the 100 faces of alpha-PdD and β-PdD. The next layer of atoms is applied to both the alpha-phase and beta- phase with a shift of 1/2 unit cell.
The lattice parameter of β-PdD increases as additional lattice sites are occupied by deuterium. A value of 0.4025 nm is published for D/Pd = 0.61, which is the value for β-PdD in equilibrium with alpha-PdD at the lower phase boundary of the beta phase. A value of 0.405 nm has been measured for β-PdD
0.77 [204].
As many authors have pointed out, the distance between deuterium nuclei, even in β-PdD
1.0, is too great to allow fusion by a “normal” process
[205, 206]. No knowledge exists about the structure or lattice size of phases above β-PdD
1.0 or of nanosized particles. In addition, no knowledge exists about the complex alloy phases that are actually on a the effect of ”normal” processes can not be evaluated.
II.3. Thermodynamic properties
The thermodynamic properties of beta-PdD are very similar to those of β-PdH, for which more data are available
[186, 207-209]. The partial enthalpy of formation for deuterium has proposed to become less negative as D/Pd increases, going positive above about 0.85
[210, 211]. This behavior does not mean that higher compositions can be achieved by simply increasing the temperature once PdD
0.85 has been exceeded
[7]. This can not happen because the entropy also changes. As a result, the Gibbs energy of formation, which determines stability with respect to the gas phase, continues to show decreasing stability as temperature and composition increase. Hence, deuterium will be lost to a gas held at constant pressure as temperature is increased regardless of the D/Pd ratio. The following equation gives the D
2 pressure over b-PdD as a function of temperature and composition, where r=D/Pd ratio and T = °K
[189].
ln P [D2, atm] = 12.8 + 2ln [r/(1-r)] - [11490-10830 r]/T
The pressure within the a-b two -phase region is given by:
ln P [D2, atm] = -4469/T + 11.78.
On the other hand, if a nuclear-active phase exists with a composition greater than β-PdD1.0, it could become more stable with respect to β-PdD as temperature is increased, because of its greater entropy. This increased concentration would provide more sites in which nuclear reactions could occur and could explain the positive effect of temperature. Of course, this ideal situation does not exist in the surface region, where the NAE is proposed to occur, because a Pd-D phase in this region, if it exists at all, is highly contaminated by other elements. Nevertheless, the nuclear effects show a positive temperature effect.
II.4. Measurements of D/Pd ratio
The deuterium content has been measured using change in resistivity, change in weight, and production of orphaned oxygen. X-rays reflected off the surface have allowed measurement of the lattice parameter, which can be used to determine the composition. All of these methods, except perhaps the latter, measure an average composition of the sample, not the composition of the NAE. Furthermore, this average will depend on the shape and size of the sample, and on the magnitude of the concentration gradient. Unfortunately, the reported values have only a general relationship to the composition of the NAE.
II.4.1. Resistivity
Many studies use the resistivity of a palladium cathode to determine its D/Pd ratio. This method gives an average composition of the sample and is influenced by many variables
[212, 213]. Composition is calculated using the resistivity ratio of sample/pure Pd (R/Ro). The value goes from 1.0 at pure Pd to about 2.0 at the lower phase boundary of the beta phase, with a linear relationship between these two end members. R/Ro decreases within the single-phase region to about 1.0 at the upper boundary, whereupon a change in slope is observed
[214]. Resistivity behavior is different for thin films
[215, 216], depending on thickness below 100 nm. This will be especially true when a thin film of palladium is loaded by electrolysis, because the highly loaded surface region makes up such a large fraction of the total in such a sample. Therefore, the resulting resistivity will more closely represent properties of the surface rather than the interior. Therefore, values obtained from thin films can not be compared to those obtained from bulk material where the value is determined mainly by the much lower interior composition.
Resistivity in the two-phase region between alpha-PdD and β-PdD should be a linear combination of values between the end members. However, because hysteresis effects occur in this composition region in the absence of equilibrium, the observed relationship may not be linear or reproducible. The maximum value for R/Ro at the low boundary of the beta phase is determined by the composition acquired by β-PdD. This composition is affected by temperature, pressure and impurity content, hence does not have a unique value. However for convenience, people use a complex polynumeral to define the behavior between pure Pd and β-PdD1.0, a method that may introduce significant error in the low composition region of the beta-phase. Furthermore, once PdD1.0 has been exceeded, the resistivity must assume a different behavior determined by another two-phase region. Consequently, the behavior can not be extrapolated beyond PdD1.0.
II.4.2. Weight change
When loaded to PdD1.0, a 1g sample of Pd has increased in weight by 0.0185 g. As a result, composition can be determined using a 4 place balance. Because such samples deload rapidly, measurements must be made as a function of time and extrapolated back to the time when electrolysis was stopped using the square root of time.
II.4.3. Orphaned oxygen
When D2O is electrolytically decomposed and D2 reacts with palladium, orphaned oxygen remains behind as a gas. The amount of this gas can be used to determine the amount of deuterium added to the cathode, provided a recombiner catalyst is present in the cell. This can be done by measuring the pressure increase in a sealed system or by observing how much fluid is displaced from an external reservoir. The method, when calibrated after the study by measuring the weight change of the cathode, has the ability to measure D/Pd to ±0.005 during loading.
II.4.4. X-ray lattice parameter
This method is difficult to apply to conventional cells, but can be effective in measuring the near-surface composition when X-rays can be reflected from the surface.
CHAPTER 6: How to reproduce the Pons-Fleischmann effect
Because so many methods have produced anomalous effects, a researcher wishing to replicate the cold fusion effect is faced with a dizzying array of choices, and must begin by choosing a method. He/she must also choose which anomalous effect -- heat, tritium, helium, or transmutations -- will be used to gauge success. Unfortunately, the methods of doing cold fusion and measuring success require skill and measurement of the effects with confidence requires expensive instruments. Regardless of how simple the Pons-Fleischmann method looks, it is a complex and difficult experiment not suited to the amateur.
Nevertheless, of the methods used, the Pons-Fleischmann Effect requires the least expense to investigate, although its replication is considered difficult. Detection of anomalous heat is the most cost-effective way to reveal anomalous behavior. However, if suitable tools are available, measurement of transmutation products on the cathode surface frequently produces fewer ambiguous results. Improved success is achieved when additional energy is added to the cathode in the form of plasma that is generated using pulsed high voltage or laser heating.
Past efforts to duplicate the P-F Effect concentrated on the bulk properties of the palladium cathode
[217, 218]. Ways were found to reduce cracking and achieve a high average D/Pd ratio. While these methods were sometimes successful, greater success can be achieved by concentrating on the nature of the deposited surface, regardless of what is used as the substrate. Palladium can be plated on clean platinum
[18] or on silver outside of the cell, or a suitable coating of palladium can be applied to an inert substrate
in situ using an electrolyte containing PdCl
2 +LiCl
[219]. This method has enjoyed frequent success. Celani
et al. [253] have produced high compositions in thin films and fine wires of palladium using a very dilute electrolyte containing SrCl
2+HCl+CO
2 and a small amount of HgCl
2.
Efforts must be made to insure that the electrolyte contains the correct impurities and does not contain “bad” impurities. The nature of “bad” impurities is not well understood, causing some batches of heavy water not to work for unknown reasons. A new cell is best cleaned using a dummy cathode as a getter that is removed after several days of electrolysis and replaced by a new cathode. If a suitable impurity layer is not applied outside of the cell, a layer of microcrystals must slowly form on the cathode surface during the study. When such a layer is applied within a conventional P-F cell, it consists mainly of lithium from the electrolyte, silicon from the Pyrex, and platinum from the anode. This deposition process will be very slow because platinum becomes available only as rapidly as black platinum oxide forms on and dissolves off the anode surface. This process is accelerated by the presence of Cl
- in the electrolyte. Pyrex slowly dissolves in the electrolyte and this process is accelerated as the lithium content of the electrolyte is increased. As a result, considerable time must pass before success is achieved in a new cell. In fact, attempts to duplicate the effect using very pure materials contained in Teflon cells failed until Pyrex was added to the solution. Other chemicals can be added, such as aluminum
[220] or thiourea
[221, 222], which will sometimes produce an active coating and/or higher composition more rapidly. With this new understanding, the challenge must focus on placing suitable materials in the electrolyte at a low concentration so that small crystals will grow on the surface. Too high a concentration of impurities within the electrolyte will not work because the crystals will rapidly grow too large to be useful.
CHAPTER 7: Theory
I. Introduction
Hundreds of attempts have been made to explain the CF effect. A broad range of possibilities have been explored, but with little success. So far, no theory has successfully shown how the effect can be amplified and made more reproducible, even though many suggestions have been made. This failure has resulted from an emphasis on the nuclear mechanism instead of on the environment in which the reactions occur. The experimentalist has control only over the environment, with the nuclear mechanism occurring only after this environment has been created.
A successful theory must meet several basic challenges. First, a mechanism must be found to overcome the Coulomb barrier of hydrogen as well as elements having much larger barriers. Second, once nuclear energy is released, a mechanism must be found that can quickly degrade the energy to prevent emission of significant energetic radiation, which is not detected. Third, a unique environment must be identified and shown how it influences the nuclear mechanism, especially how it determines which of the many possible nuclear reactions is catalyzed. Fourth, formation of helium without gamma emission needs to be explained. Most theories address only one, or at most two, of these challenges. Until a theory can show how the NAE is created and can describe its unique nature, little progress will be made, especially because most theories are based on the ideal properties of β-PdD. As explained in Chapter 4, the NAE does not involve this compound under many conditions, if at all.
II. General Discussion
II.1 Role of neutrons
Obviously, if neutrons are involved in the nuclear mechanism, the Coulomb barrier would not be an issue. Therefore, many people have proposed a source of potentially reactive neutrons. A few of these theories are described here.
Kozima
[223] has written a large number of papers based on the idea that neutrons are contained in normal materials in stabilized form. When proper conditions are created, i.e. the NAE, these structures become unstable and react with surrounding nuclei. He calculates the concentration of these “clusters” and uses consistency of the resulting value as support for the idea.
Fisher
[224] proposes that neutron clusters are lightly bonded to certain nuclei. When proper conditions are created these clusters breakup and react with other nuclei in the environment. Evidence for super-heavy carbon, presumably caused by an attached neutron cluster, has been reported by Oriani
[225].
Many people have observed that if the electron associated with hydrogen or deuterium could get sufficiently close to the nucleus, a virtual neutron or a dineutron would result. In this way, the electron might provide enough shielding for the proton or deuteron to enter a nucleus. Presumably, the electron would not have to actually create a real neutron, a process that requires energy and a neutrino. Mills
[226] provides a theoretical basis for allowing the electron to closely approach the nucleus, with the formation of the so-called hydrino. Dufour
[227] has made a similar suggestion. Both people have provided evidence for the shrunken hydrogen concept.
All theories based on real neutrons must explain how the NAE releases the neutrons or causes their creation, and why so few neutrons escape from the active region, even though this region is too small to offer much absorption. Formation of 4He without 3He or tritium production also needs to be addressed.
II.2 Role of phonons
A phonon is a mythical particle used to describe energy contained in the vibration of atoms and electrons located within a material. These vibrations are proposed to cause a few atoms to approach one another within nuclear reaction distance
[228, 229] or to cause energy to accumulate within a nucleus
[230] so that the nucleus becomes unstable. Once a nuclear reaction releases energy, phonons are proposed to communicate this energy to the lattice
[231]. Besides the considerable challenge of showing that phonons have the necessary properties to do the proposed jobs, it is necessary to show why this only happens within the unique NAE.
II.3 Role of particle-wave conversion
The Chubbs
[232], have proposed that the deuteron nucleus can, under proper conditions, convert to a wave. As such, it can interact with another deuteron wave without a Coulomb barrier being present. This interaction briefly forms a helium wave, which slowly converts to a helium particle by losing small quanta of energy to the surrounding lattice. This model solves a few problems, but it does not account for how transmutation products are produced and what unique property of the lattice encourages this conversion. Simply having a periodic array of atoms is not sufficient because this condition exists throughout the material while nuclear reactions are localized in special regions.
II.4. Role of “Strange” particles
Explanations based on rare particles have been proposed. These include the Erzion
[233], the NATTOH
[234], fractionally charged particles
[235], massive negative particle
[236], and superheavy nucleus
[237]. How these particles are activated by or impact on the NAE is not clear.
II.5 Role of tunneling or enhanced cross section
A number of authors have explored the possibility that processes within the PdD lattice might reduce the effective Coulomb barrier. Only two of the initial suggestions are cited here. The processes are proposed to involve mechanisms that bring the D atoms closer together than normal using resonance effects
[238], or processes that introduce electron screening between the D atoms
[239]. Most models fail to show what makes PdD unique in supporting a fusion reaction and they do not address other kinds of nuclear reactions. In general, the proposed models have not been able to explain the rate of fusion required for anomalous heat production or heavy element transmutation.
Recently, a source of screening electrons has been suggested to exist between two materials having different work functions, the so-called swimming electron theory
[240-242]. This model is consistent with conditions existing in the apparent NAE and addresses the formation of heavy elements.
II.6. Role of multi-body fusion
Multi-body fusion was first suggested by Takahashi
et al. [243] who arrived at this model using the energy spectrum of neutrons being emitted from an electrolytic cell. Later studies using ion bombardment are consistent with the model
[244]. Recently, Iwamura
et al. [23] show evidence for 4 deuterons entering a nucleus simultaneously, adding additional support to the multi-body model. Formation of such clusters
[245] followed by fusion within the cluster solves many problems, not the least of which is a method to release momentum without emitting a gamma ray. In this case, energy is deposited in the lattice by energetic alpha particles and deuterons ejected from the cluster. The challenge for this model is to show how such clusters can form in a lattice and the nature of such a lattice.
CHAPTER 8: Suggested errors and prosaic explanations
Skeptics suggest that all cold fusion results are experimental error and instrument artifacts. To prove this hypothesis, they would have to examine each well-written, detailed cold fusion paper to find a set of errors that can explain away all observations. As difficult as it is to explain the nuclear reactions, finding such a comprehensive set of errors is even more difficult. Furthermore, it would call into question the validity of the experimental method itself. To reduce the challenge, most skeptics proposed an error that might occur in one study and then assume it applies to all other studies. They do not try to examine each study and they fail to realize that different instrument types and techniques are used that rule out the possibility of the proposed error occurring elsewhere. For example, skeptics often suggest that recombination may explain marginal excess heat in an open cell experiment, and then apply this critique to closed cells in which a recombination error is impossible
[246]. Or they assume a prosaic process they can imagine to occur without offering any proof that the process actually occurs in nature. From a skeptic's point of view, the rules of evidence apply only to the person making a claim, with a suggested error requiring no justification whatever. While this approach is not very constructive, serious errors do occur in any experiment and these need to be identified.
A number of real errors have been identified, which will be examined in detail below. Many more have been discussed by Storms
[71].
Temperature gradients within an isoperibolic calorimeter
The first criticism of the Pons-Fleischmann heat measurement was based on a presumed artifact caused by temperature gradients within their isoperibolic cell
[247]. P-F showed that these were absent by moving their thermistor to different levels within the cell
[248]. Nevertheless, this is a valid potential error
[67]. Electrolytic stirring is seldom sufficient to completely remove the temperature gradient. Even mechanical stirring must be held very constant to achieve a stable measurement. Because of this potential error, most recent work uses either flow calorimetry or a Seebeck calorimeter, both of which do not suffer from this problem. A second-wall isoperibolic calorimeter has also been used with success.
Changes in calibration constant
All calorimeters must be calibrated. The resulting calibration constant may not always remain constant. Each time such a measurement is made, slightly different values are always obtained. If the claimed anomalous energy is within the range defined by many calibrations, its reality can be questioned. Shanahan
[249] argues that all claims for anomalous heat are caused by an unexpected change in the calibration constant because of some undefined process within the cell, but by not a nuclear reaction. An answer to this challenge rests on three facts:
(3) anomalous heat sometimes is associated with helium production or transmutation products, which are clear indicators of a nuclear reaction.
Furthermore, a process that can produce such a change in all calorimeters has not been demonstrated, only suggested. At this time, if a person wants to reject anomalous heat, the proposed prosaic process must be demonstrated using as much rigor as was used in making the initial claim, not simply suggested.
Tritium contamination
When anomalous tritium was first claimed, it was rejected based on tritium being present in the environment, the cell materials, or in the palladium. All of these sources have been carefully analyzed and found to be free of sufficient tritium. At the present time, no plausible source has been found that explains all of the observations, other than its creation by a nuclear reaction.
Air contamination in helium
Early claims for helium production were rejected because of a presumed leak of air into the container. Since then, people have shown that air is absent by measuring the Ar content at the same time as helium is measured. This eliminates this possibility. While this is a difficult measurement, requiring a complex instrument, the growing number of measurements showing a relationship between helium and energy makes a rejection of this relationship increasingly implausible.
Heavy element contamination
Detection of stable elements is difficult when very low concentrations are present, although such measurements are seldom questioned when applied to conventional research. In addition, practically everything contains a small amount of most other stable elements. Therefore, proving that a particular element, after having been concentrated on a cathode by electrolysis, has a nuclear origin can be tricky. A plausible claim is usually based on a large and unexplained concentration being present or an abnormal isotopic ratio. Some work even shows how the abnormal element increases with time. Clearly, most stable elements found on a cathode are not abnormal. However a sufficient number of observations for the presence of anomalous elements has been reported to make the transmutation reaction worthy of study.
SUMMARY
The following are proposed as new insights provided by recent observations described in this paper:
1. Pure β-PdD, regardless of its deuterium content, is not the environment in which LENR occurs during the Pons-Fleischmann effect. The complex alloy in which the effect occurs apparently requires a very high deuterium content.
2. Nearly pure β-PdD appears to be active at a relatively low deuterium content.
3. LENR requires nanosized particles of various complex materials, including living cells.
4. All isotopes of hydrogen can be involved in LENR.
5. Clusters of hydrogen isotopes form and interact with each other and with nearby nuclei to cause LENR.
6. Many elements can enter into LENR either with hydrogen or with each other.
These conclusions are significantly different from conventional thinking in the field and well outside of what conventional physics can explain. Hopefully, rather than being rejected, these aspects of the phenomenon will be considered when new experiments and explanation are attempted. So far, present experiments and theories have not been very successful, so a person has little to lose by considering these possibilities.
COMMENTS
Science has been successful because certain rules of evidence were adopted centuries ago, the so-called Scientific Method. These rules require that many people using different devices duplicate all novel observations. Such replications reduce the human tendency to deceive and to be deceived. In addition, the behavior observed in these various studies must show similar patterns, i.e. important variables must have the same effect in all studies, regardless of the equipment used. Having an explanation for the strange behavior is NOT initially necessary, although eventual discovery of an explanation is important. This is a good method and has served mankind well when it is faithfully applied. Science fails when these rules are ignored. They can be ignored several different ways, the most obvious being premature acceptance. Some scientists think this rule so important that they base their careers on protecting Science from such a violation. A less obvious problem occurs when repeated replications are ignored because a scientist does not WANT to believe a result that conflicts with a favorite theory. Initially, cold fusion was rejected for the former reason. Now rejection is based on the latter. The first rejection was valid and consistent with the Scientific Method. The present rejection is not.
Skepticism, when carried to extreme, is as damaging as naive acceptance. At the present time, many people respect the skeptic for guarding the high ideals of science. In fact, skeptics frequently stop important progress, stifle originality, and turn creative people away from science altogether. Although many examples of this injury can be cited from the past and especially from the present time, this rejection of cold fusion is particularly egregious because of its vehement nature and the importance of the discovery. I ask you, the reader, to use good judgment and a responsible attitude in evaluating the incredible claims described in this Guide. Remember that new and strange claims do not have to be blindly accepted or blindly rejected, just explored with an open mind. Important new ideas almost always conflict with conventional understanding, so such conflict should not be used as a basis for outright rejection, before the possibilities have been carefully examined.
Attached:
A critique of this paper by Kurt Shanahan, plus rebuttals by Edmund Storms and Michael Staker
An Addendum to this paper was added in March 2011, Storms, E., What is now known about cold fusion? (Addendum to Student's Guide). 2011, LENR-CANR.org.
Source: